There are two reasons the headline of this article may have caught your attention. Either you’re one of the estimated 53 percent of people with SCI who experience neuropathic pain, or you’re one of a growing number of Canadians turning to cannabis as a prospective salve to improve sleep, reduce anxiety or, you guessed it, treat chronic pain.
If you fall into the first group— we know there are a lot of you out there—and you’re one of our dedicated readers, you probably already know that neuropathic pain is a frequent topic of conversation for us. We’ve covered everything, including cognitive therapies, physical exercise, skin creams and medications, all in our effort to support the SCI community with evidence-based solutions to the problem of chronic pain. While medications, including antiepileptics, antidepressants and opioids are the most common treatments for this problem, the potential for pain relief must often be balanced with a host of negative side effects, ranging from dizziness and drowsiness to drug dependence.
We also know that many of you fall into the second group. You’ve typed “cannabis and chronic pain” into the search bar of your web browser or talked to a friend who swears by cannabis for pain relief. Perhaps you’ve even used cannabis as a way to successfully manage your own pain.
The problem is that (despite what the internet, your friend, and your personal experience might suggest) we don’t have any consistent scientific evidence to show that cannabis is an effective treatment for neuropathic pain following SCI. And without a strong body of evidence, finding support for cannabis as a pain treatment—from medical prescriptions to insurance coverage—as well as guidance for a safe and effective dosage, can pose a significant challenge. But thanks to a new trial underway in Australia, we might have that evidence as early as 2024.
In partnership with the Lambert Initiative for Cannabinoid Therapeutics, a group of researchers led by the University of Sydney’s Professor Luke Henderson was awarded $1.7 million Australian dollars (approximately $1.5 million Canadian) from the New South Wales government to examine if cannabidiol (CBD), the non-intoxicating component of cannabis, is safe and effective for reducing neuropathic pain in people with SCI. The trial also aims to tease out the possible reasons why chronic pain develops, and the potential ways CBD might improve it.
“If effective, this trial will provide gold-standard evidence to support the use of CBD to patients with neuropathic pain following spinal cord injury,” says Dr. Anastasia Suraev, the trial coordinator and a Research Fellow in the School of Medical Sciences at the University of Sydney. “It can also help to inform and ultimately change policy surrounding the prescription of medicinal cannabis for the treatment of neuropathic pain and improve patient access.”

Globally, this trial is the first of its kind. While research indicates that CBD is a promising treatment option for neuropathic pain, the strength of the evidence to date is lacking.
For example, research with animals shows that CBD is an effective way to reduce pain hypersensitivity that develops during neuropathic pain. In addition, a recent trial suggests that nabiximols (sold under the brand name SativexTM), a mouth spray containing equal parts CBD and tetrahydrocannabinol (THC), the psychoactive component of cannabis that produces the “high” sensation, can reduce neuropathic pain compared to a placebo (a substance that does not contain the active ingredient, but appears identical to the active drug).
But when looking at CBD alone as a treatment for neuropathic pain in humans, Dr. Suraev warns that the findings of the research must be heeded with caution. While it’s possible that CBD may be an effective way to reduce (and possibly prevent, in the case of cancer patients going through chemotherapy) neuropathic pain, the way that existing research was designed and carried out increased the likelihood of bias and placebo effects. In other words, the effects of the CBD may have been due to the participant’s belief in CBD as a treatment for neuropathic pain, rather than the CBD itself.
“In fact, no randomised, double-blind, placebo-controlled trial—the most rigorous way to test the potential benefit of a medication for a specific condition— has been conducted to-date exploring the efficacy and safety of oral CBD in the treatment of neuropathic pain, let alone neuropathic pain following SCI,” says Dr. Suraev.
What this means is that neither the participants nor the researchers in the trial know who is getting a placebo and who is getting the active drug, and that participants are randomly assigned to a treatment group, limiting the influence of either the participant’s or the researcher’s belief about the treatment’s effectiveness. The effectiveness of the treatment is then compared against the placebo to find out how well the treatment worked. This is the “gold standard” of evidence that the University of Sydney-based research team seeks to achieve.
According to Rebecca Robertson, a PhD Candidate in the School of Medical Sciences at the University of Sydney focusing on the study of neuropathic pain following SCI, the trial began in June 2022 and it will run until mid-2024. Participants will get to trial both CBD and a placebo in random order over two six-week periods, and each period will be separated by a four-week “washout” to make sure the first treatment (CBD or placebo) is no longer in their system.

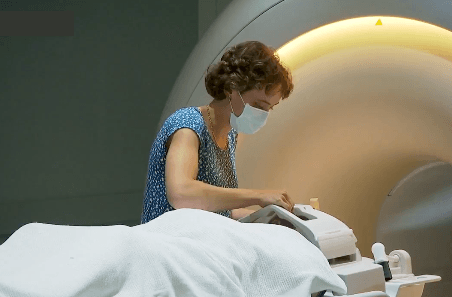
“During each treatment period, participants will be asked to rate their pain and wear a wrist-worn device that measures their sleep,” says Robertson. “Before and after each treatment period, participants will be asked to attend Neuroscience Research Australia (NeuRA), a leading medical research institute in brain and nervous system research, for a magnetic resonance imaging (MRI) and electroencephalogram (EEG) scan, questionnaires about their pain, sleep and mood, and a blood test.”
The research team will use the MRI and EEG scans to examine functional, structural and chemical changes in participants’ brains over the course of the trial. Likewise, they will use the blood tests to examine changes in participants’ blood after each treatment period. These measures will be combined with participants’ self-reported pain, mental wellbeing, and sleep to assess if, how and why the CBD treatment works or doesn’t work when compared to the placebo, says Robertson.
“Together, these measures will allow us to build a holistic picture of the changes that may underly an effective chronic pain therapy, and why it is or isn’t effective—with the main goal being a reduction in pain during the CBD treatment,” she explains.
Because CBD is non-intoxicating and has relatively few side effects (even at high doses), it is considered an ideal long-term treatment, says Robertson. “The main concern related to CBD’s safety is the potential for drug-drug interactions that might occur when CBD is co-administered with standard treatments,” she explains. “However, this can be managed with slow increases in dosage and careful monitoring.”
Ultimately, the trial will aim to identify and understand mechanisms that lead to neuropathic pain after SCI and predict individual responses to CBD treatment. For example, the research team hopes to identify biomarkers that will indicate who is most likely to benefit from CBD treatment, says Robertson. If the CBD treatment is effective, the trial has the potential to provide individualized, evidence-informed guidance around the dosage of CBD that can safely and effectively treat an individual’s neuropathic pain.
The trial could also lay the foundation for advancements in neuropathic pain research more broadly, claims Dr. Suraev. “The potential wider impact of this trial is a deeper understanding of the underlying causes of chronic neuropathic pain (with or without SCI) which could lead to even further refinement of therapy,” she explains. “Success of this trial would trailblaze future research on the benefits of CBD in other neuropathic pain conditions which, much like neuropathic pain following SCI, remain undertreated and misunderstood.”
Preliminary findings of the trial will be available in late 2024 or early 2025, says Robertson. Until then, we’ll have to wait for a better understanding of the role that CBD can play, if any, in neuropathic pain treatment. Stay tuned—we’ll keep tabs on how the study progresses and report back when the results are in.
This article was originally published in the Summer 2023 issue of The Spin. Read more stories from this issue, including:
- Adaptive paragliding
- Artificial intelligence
- Partner exercise
And more!
Read the full Summer 2023 Issue of The Spin online!