The buzz keeps growing about the potential of neurostimulation to restore function for people with SCI. Is the hype warranted? ICORD’s Dr. Tom Nightingale helps us put it all into perspective.

An Australian show recently featured Dr. Bryce Vissel, a respected Professor of Neuroscience at The University of Technology Sydney, and his efforts to convince the Australian government to invest millions in order to make neurostimulation available to every Australian with SCI. The show resulted in media attention around the world.
Here at SCI BC, we are also bullish on neurostimulation. In the past couple of years, we’ve published numerous research stories about neurostimulation, which involves electrically stimulating the damaged area of the spinal cord in order to reawaken dormant nerves and restore function. Neurostimulation’s apparent ability to restore some level of walking or stepping function has garnered most of the mainstream media’s attention, but we are particularly intrigued by its promise of restoring many less glamorous but critical functions in people with chronic SCI—for example, hand, bowel, bladder and cardiovascular function.
But is neurostimulation ready to be thought of a potential cure for SCI, as suggested by Professor Vissel? Should researchers in Canada be spearheading a similar effort? Or is the hype overshadowing the reality? Is the excessive focus on walking overshadowing its potential to make improvements in areas considered much higher priority by people with SCI themselves, such as better bowel, bladder and hand function?
Recently, we posed these and other questions to Dr. Tom Nightingale, a postdoctoral research fellow working in the autonomic function laboratory of ICORD principal investigator Dr. Andrei Krassioukov.
Nightingale, who is originally from the UK, is greatly interested in using neurostimulation strategies to restore autonomic function following SCI—in fact, he’s currently a co-investigator on a joint ICORD and University of Calgary transcutaneous spinal cord stimulation clinical trial that will soon be open for enrollment, and is involved in other neurostimulation projects as well.
Relying on his expert assistance, our goal was to try to put the current state of neurostimulation research into perspective for our readers; to sift through the hype and gain a better understanding of its true potential to restore function and quality of life for people living with SCI.
Q. What prompted you to write your piece for The Conversation, a respected not-for-profit global media outlet?
A. I was amazed by the media buzz generated by the two case-series published in Nature and New England Journal of Medicine demonstrating that neuro- stimulation, coupled with intensive physical therapy, allowed participants with SCI to walk again.
While extremely exciting, this media hype is seemingly inconsistent with consumer priorities. We have known for some time that certain autonomic functions—for example, blood pressure control, bowel, bladder and sexual function—are of a higher priority for everyday quality of life 2019 studies confirmed that restoration of bladder, bowel and sexual functions are of utmost importance for people with SCI.
The Conversation piece showcased pioneering work from our laboratory and others that demonstrates neurostimulation has the potential to safely and effectively restore crucial autonomic functions. We are currently following up these interesting preliminary observations with larger clinical research trials, enrolling a larger number of research participants.
Q. There seem to be many study results confirming efficacy of neurostimulation. Given that, is there a growing confidence among the research community that this truly represents a leap forward in terms of providing a real world solution to improve the lives of people with SCI?
A. Exciting data exists showing neurostimulation can help to restore critical functions for people with SCI. You would be hard-pressed to find an existing therapy that has such promising acute effects as neurostimulation for restoring certain functions—for example, modulating blood pressure, resolving orthostatic hypotension (dangerously low blood pressure), improving lower urinary tract function and substantially reducing the time needed for bowel management. These are seemingly significant functional improvements that would undoubtedly lead to enhanced quality of life.
Emerging evidence is now starting to demonstrate that, after a prolonged period of daily repetitive stimulation is concluded, these improvements persist, indicating long-term benefits.
There was a heavy neurostimulation focus at the premier North American SCI conference, suggesting that researchers are excited and intrigued by the therapeutic potential of such strategies.
What is neurostimulation?
Neuromodulation is defined as “the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body.” Neuromodulation therapies, in common use since the 1980s, can help restore function or relieve symptoms that have a neurological basis.
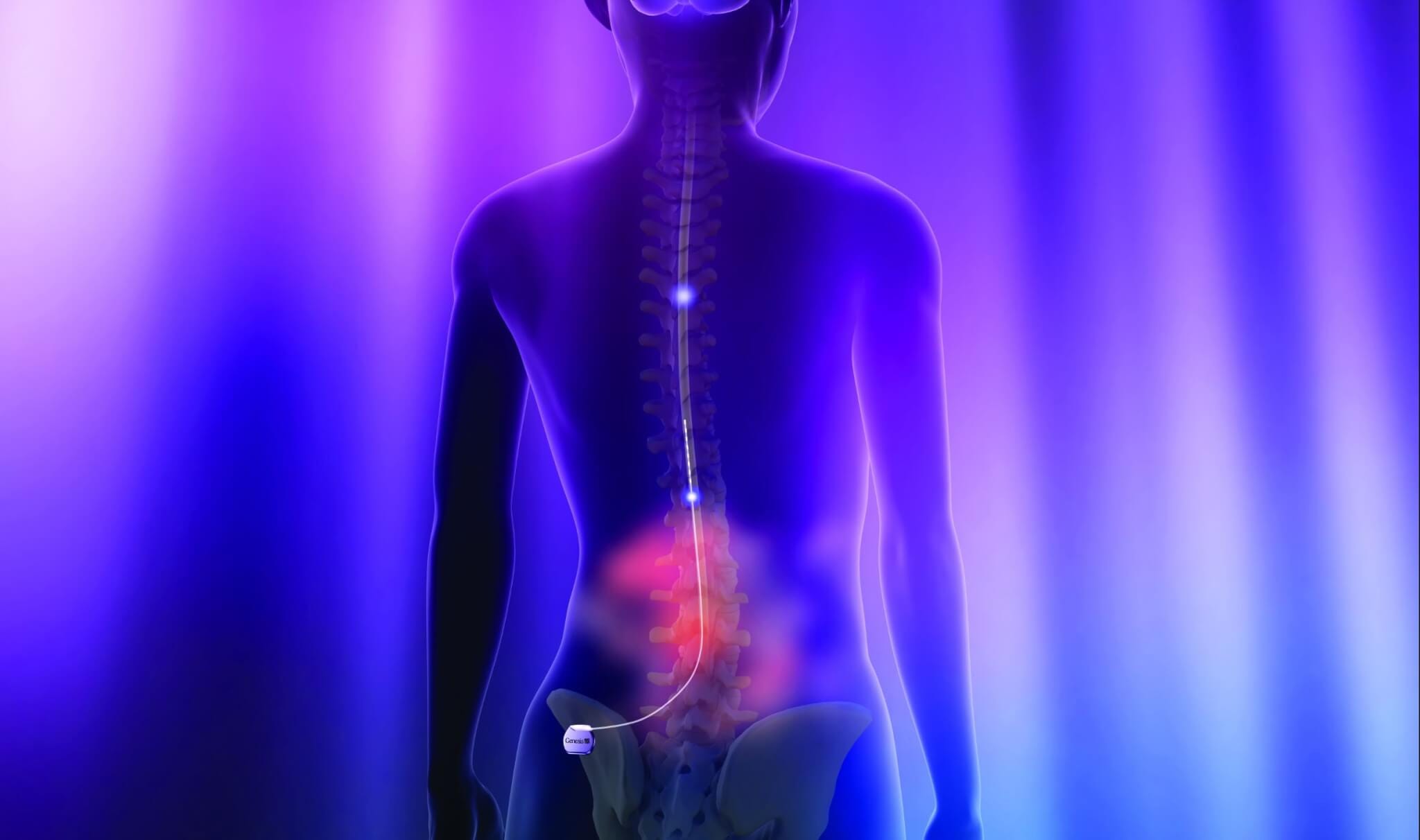
Neurostimulation is a form of neuromodulation and refers to techniques that rely on electrical or electromagnetic stimuli to modulate the target nerves within the body. Typically, neurostimulation involves a transmitter (control unit and pulse generator) and electrodes. The transmitter emits electric impulses, which are delivered to the target area via electrodes.
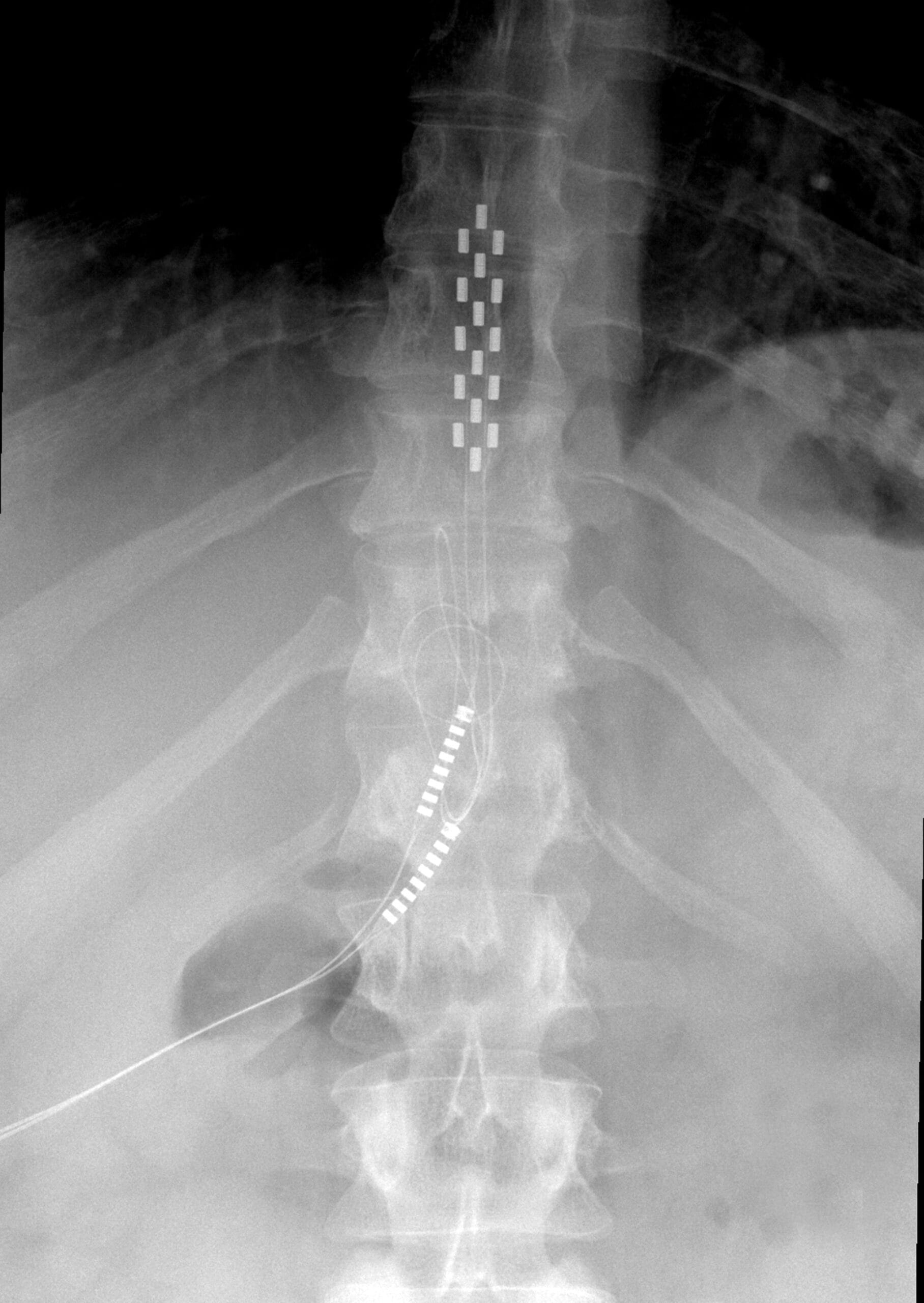
There are two primary forms of neurostimulation being investigated for use with SCI: epidural and transcutaneous. Epidural approaches are known as invasive, as they involve surgically implanting a stimulator and electrodes into the space around the dura mater of the spinal cord. Transcutaneous approaches are non-invasive, as they rely on an externally-worn transmitter and electrodes adhered to the skin above the target areas of the spinal cord.
Unfortunately, the number of participants enrolled in these studies is small. For example, in epidural (invasive) neurostimulation, it’s pertinent to remember that only approximately 40 individuals with SCI have had an epidural simulator implanted worldwide. Therefore, while the optimism is warranted based on exciting preliminary data, it is perhaps wise to be somewhat cautious and realistic. To date, some other treatments that have showed promise in pre-clinical research either did not reach clinical trials or failed to demonstrate therapeutic benefits at the clinical stage.
Consequently, monumental efforts by researchers such as Bryce Vissel to secure a substantial amount of government funding to investigate these emerging technologies is necessary to further advance the field.
Q. What are your thoughts on the two primary approaches to neurostimulation for SCI—the surgically-implanted epidural approach, and the transcutaneous approach, such as the pioneering work of Dr. Reggie Edgerton and his efforts to develop a device and bring it to market?
A. The transcutaneous neurostimulation paradigm specifically may offer a simpler, less risky and more cost-effective solution. This approach uses conventional electrodes and stimulators that already have a well-established safety profile.
In contrast, despite utmost care and the highest surgical standards, patients undergoing surgical implantation are at potential risk of surgical complications. Although rare, there are reports of implanted electrodes leading to epidural hematomas in chronic pain patients, with a potential risk of spinal cord damage and paralysis due to compression.
Therefore, less invasive devices may represent a more accessible real-world option that considers patients’ comfort and safety. Transcutaneous spinal cord stimulation has the potential to emerge as a widely-used therapy as it provides similar neuromodulatory effects as more invasive stimulation, without the need for surgery and permanently implanted devices.
Q. Can we ask you to do a very unscientific thing? If you’re comfortable, please speculate about how confident you are that this technology may offer a real benefit at some point in the near future for our readers.
A. Transcutaneous stimulation, which is a non-invasive, routine procedure that uses conventional electrodes and isolated current stimulators, is already approved by some health regulatory authorities, including the USDA and Health Canada, for various indications such as transcutaneous electrical nerve stimulation (TENS) to treat pain. Although displaying a good safety profile, the specific for motor control, lower urinary tract, bowel and sexual functioning, remain to be approved. As a conservative estimate, I believe it will be possible to be approved and accessible to readers within the next decade. On the other hand, I think it’s challenging to speculate on if and when epidural stimulation might be a commonplace procedure for individuals with an SCI.
Larger-scale, pivotal studies are essential prior to receiving health regulatory body approval and translation into clinical practice for specific indications.
On a positive note, some funding agencies, such as the Craig H. Nielsen Foundation, encourage research funding proposals to include a full clinical translation plan. This includes an outline of all major project milestones that are expected and necessary to demonstrate sufficient potential as a future therapy. This streamlines the process and, along with specific translational funding opportunities such as NINDS CREATE and the NSF Innovation Corp programs, will hopefully ensure faster progress in the translation of research breakthroughs to clinical practice.
Q. Finally, what do we all need to be cautious about, in terms of realistic hopes and limitations of neurostimulation research?
A. This is a great question. I believe we are some way from optimizing these techniques for specific bodily functions. There are many, many unanswered questions.
For example, with transcutaneous neurostimulation, what is the optimal location of electrodes to restore lower urinary tract voiding and storage? Are these the same, or do they differ for certain bowel and sexual functions and across individuals? Does this technique offer the specificity provided by epidural stimulation whereby very localized parts of the spinal cord can be stimulated?
Transcutaneous stimulation itself is not without risk. Improper use can lead to skin burns or irritation. Furthermore, the precise positioning of electrodes may be problematic for individuals who have experienced a loss of hand strength and dexterity. If necessary, how feasible or expensive will it be to replace or re position electrodes? Given that SCIs can be so different, do certain neurostimulation approaches work better for individuals with specific injury characteristics— for example, those with the greatest degree of spinal cord preservation?
Regardless of the approach used, what are the best stimulation parameters—for example, intensity, pulse width, and frequency—and do these differ depending on the functional response trying to be restored? From a long-term stimulation perspective, what is the most appropriate frequency, intensity or duration of stimulation, and does this differ depending on the specific indication? What is the minimal amount of stimulation someone needs to see improvements?
I believe these are some of the key questions the research community need to address to maximize the efficacy of various neurostimulation strategies.
Visit www.icord.org for more information on current studies.
This article first appeared in our Summer 2019 issue of The Spin and has been edited for our blog. Read the full version alongside other stories, including:
- Taking a Drive Down Memory Lane
- Clear Vision: Neurostimulation Validity
- Adaptive Surfing
- and more!



